Abstract
Introduction T-prolymphocytic leukemia (T-PLL) is a rare T-cell neoplasm of aggressive clinical course and poor outcome. Treatment with the monoclonal antibody to CD52-alemtuzumab (alem) has considerably improved outcomes, yet with transient responses. Bendamustine (benda) showed encouraging results, particularly in alem-refractory patients (pts).
Allogeneic hematopoietic stem cells transplantation (Allo-HSCT) has been reported to be effective in T-PLL, yielding durable remissions and improved overall survival (OS). New approaches using well-tolerated therapies and drugs that target such growth and survival signals as that the JAK/STAT pathways or BCL2 have recently been tested in small series of pts ineligible to intensive therapy.
Methods The present multicenter study aimed at evaluating clinical and biological characteristics, as well as outcome of a cohort of 157 T-PLL pts over 18 years old (yo) treated in any of 24 centers of the French Innovative Leukemia Organization (FILO) between 03/1997 and 05/2022. OS, relapse, progression and progression-fee survival (PFS) were evaluated. Molecular analyzes searched for JAK1, JAK3, STAT5B, IL2RG, NOCH1, NOTCH2, EZH2, TET2 and TP53 mutations/deletions in 39 pts.
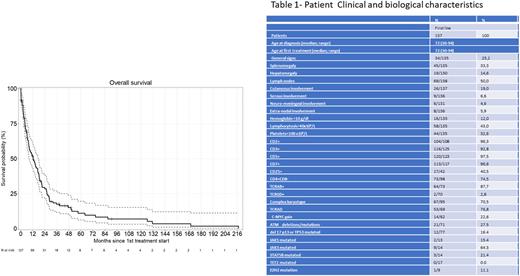
Results Pts characteristics are shown in Table 1. The median age at first therapy was 72 yo (range 30-94). Neuro-meningeal and extra-nodal involvement were present at diagnosis and first relapse in respectively 4.6% / 10.5% and 5.9% / 14.3% of pts. At diagnosis, 12% of pts had hemoglobin <100 g/L, 32.6% thrombocytopenia <100x109/L and 43% lymphocytosis >40x109/L. Flow cytometric immunophenotyping showed pan-T antigens expression (CD2,CD3, CD5, CD7) in nearly all pts (table). Alpha-beta TCR was present for 64/73 pts (87.7%) and CD25 for 17/42 (40.2%). The TPLL was CD4+ for 73/98 pts (74.5%), CD8+ for 7/93 (7.5%), double positive (CD4+/CD8+) for 15/93 (16.1%) and double negative (CD4-/CD8-) for 3/92 (3.2%).
Cytogenetics/FISH showed recurrent changes involving chromosome 14 in 69/95 (72.6%) pts [inv(14) and t(14;14) in 47/69 (68.1%) and 7/69 (10.3%)] and complex karyotype in 67/95 (70.5%).
C-MYC gain, ATM and TP53 deletions/mutations were identified in respectively 22.6%, 27.5% and 16.4% of the pts analyzed at diagnosis, while JAK1, JAK3, and STAT5B were mutated in 15.4 %, 64.3% and 21.4% of tested cases. Additional results will be presented at the meeting.
Pts received 1 to 5 lines of treatment (median 1). In first line, 45/127 (35.4%) evaluable pts received alem 30 mg IVx3 per week, after ramp-up (median:6 cycles; 1-10), with for 7 of them a previous sequence of benda (90 mg/m2 on day 1 [D1]). Thirty pts (23.6%) received first line benda 90 mg/m2 on D1 then 2x6 cycles every 28 days (median 3; 1-6), 15 (11.8%) received pentostatin, 11 (8.6%) had a CHOP-like regimen. Best supportive care was provided to 21 pts (16.5%). No treatment was proposed to 3 indolent pts (2.3%). Various schedules were applied to the remaining pts. Post-CR Allo-HSCT was performed for 20 pts (15.7%).
Median follow-ups are 9.5 months (0.03-217) for the whole cohort (Figure1) and 11.8 (0.03-221) for Allo-HSCT recipients. Median OS are respectively 12.5 (8.4-17.5) and 23.2 months (5.82-not reached [NR]). The median PFS of the whole cohort is 2.6 months (0-217). Median OS for patients treated with alem or benda in first line are respectively 14.1 (8.4-25.4) and 7.8 (4.3-21) months, while OS for pts who had a benda bridge is 8 months (3.22-NR).
The overall response rate (ORR) after first line is 42.7 % (CR 25.6%, partial response [PR] 17.1%). ORRs after first line alem or benda are respectively 53.5% (CR 41.9%, PR 11.6%) and 35.7% (CR: 14.3%, PR 21.4%).
In univariate analysis, platelets<100x109/L (HR: 1.95 [1.28-2.95]; p=0.002%) and complex karyotype (HR: 2.08 [1.19-3.63]; p=0.010) statistically significantly appear to impact survival.
Conclusion This large study demonstrates that alem therapy significantly improves the survival of T-PLL pts. Consolidation by Allo-HSCT should however be performed for all eligible patients achieving CR. Other therapeutic agents should be reserved for relapse. Prospective trials are needed to define the most effective therapeutic strategy and improve clinical responses to frontline therapy. New approaches using well-tolerated targeted therapies involving growth and survival signals are needed for the majority of patients unable to receive intensive chemotherapy.
Disclosures
Laribi:AbbVie, AstraZeneca, Beigene, Iqone, Janssen, Novartis, Takeda: Honoraria. Ysebaert:Abbvie, Astra-Zeneca, Janssen, Roche, Beigene, BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Inchiappa:ABBVIE: Honoraria; JANSSEN: Honoraria; ASTRAZENECA: Honoraria; Gilead: Honoraria. Herbaux:Abbvie: Honoraria, Research Funding; MSD: Research Funding; Roche: Honoraria; Janssen: Honoraria; Gilead: Honoraria; Kite: Honoraria; Takeda: Honoraria, Research Funding. Lepretre:AbbVie, Roche, Amgen, AstraZeneca, Janssen, Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dartigeas:Janssen, Abbvie, Roche, AstraZeneca: Other: Travel Grant; Abbvie, Roche, Janssen, Beigene, AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Willems:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: nature advantage (congress inscription) or convention; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: nature advantage (congress inscription) or convention; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Remuneration; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Remuneration. Tournilhac:IdeoGen: Honoraria, Other: Travel grant , Research Funding; Gilead: Honoraria, Other: Travel grant , Research Funding; Securabio: Honoraria, Other: Travel grant , Research Funding; Janssen: Honoraria, Other: Travel grant , Research Funding; Abbvie: Honoraria, Other: Travel grant , Research Funding; Takeda: Honoraria, Other: Travel grant , Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal